Part 2 of 2
In the previous blog, we discussed why effective hospital managers always include enhanced total cost of operation, or ETCO, as part of their equipment obsolescence plans. If you missed part one, you can read it HERE.
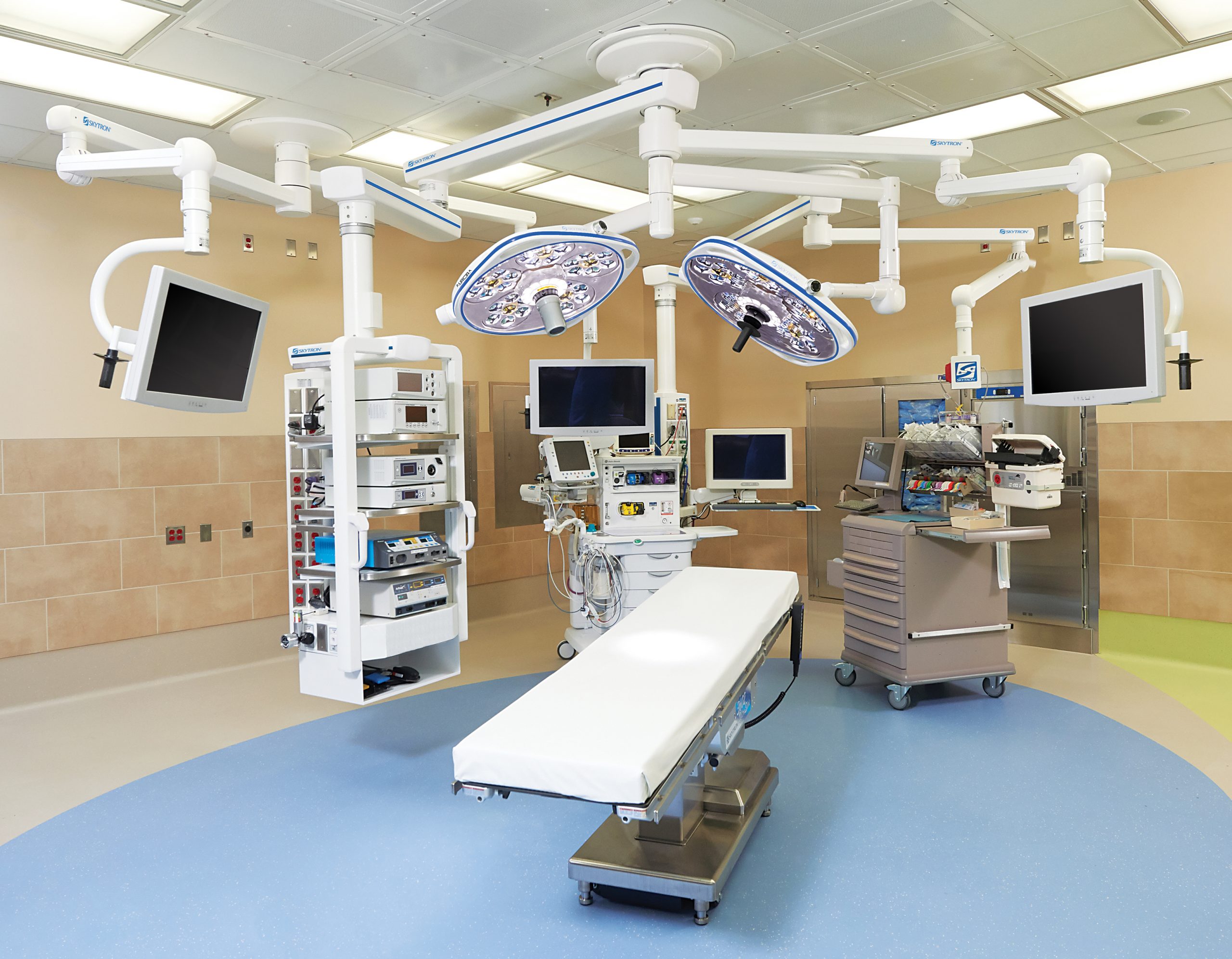
ETCO includes three components:
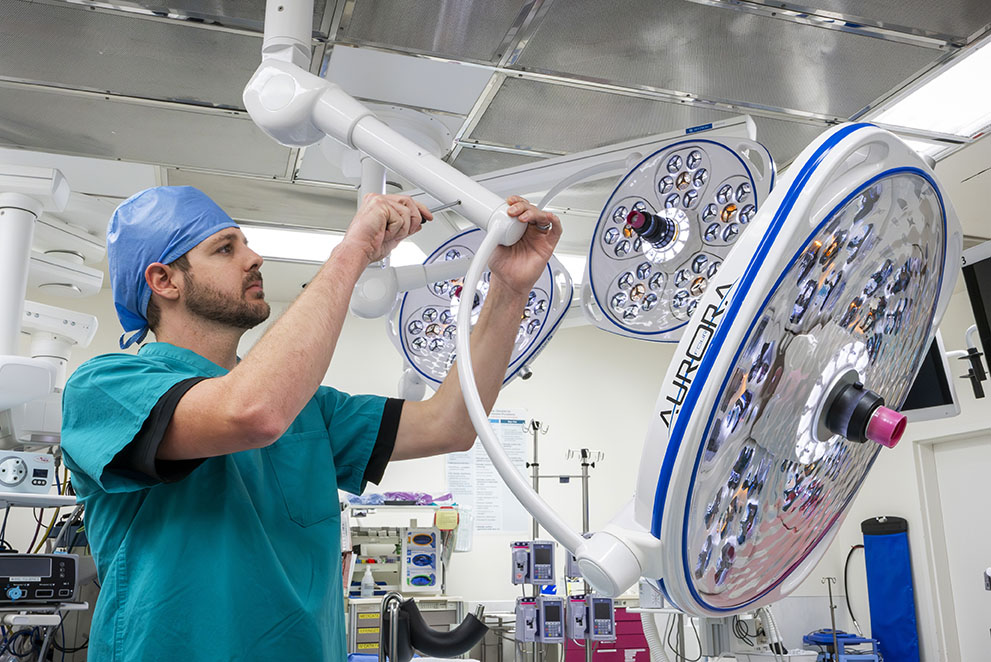
- Year-round in-service training for the staff so they understand how to properly use equipment.
- Basic equipment triage training for bio-medical staff so they can quickly troubleshoot problems and perform basic repairs, including resetting alarms that halt equipment operation.
- Preventative maintenance service plans for each piece of equipment to ensure that it is tuned up and meets manufacturing warranty guidelines.
All three components of ETOC will stop costly and unwarranted manufacturer service technician work tickets. The ETCO section of the equipment obsolescence plan reinforces how all equipment is respected by an A+ department manager. When it comes to budget decisions and equipment ROI, A+ managers typically have the edge and tend to win more than their share of capital equipment budget decisions.